Alarming new claims suggest that the GM diet is affecting animal health - prompting fears over human safety
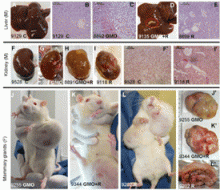
At first glance the frozen bundles could be mistaken for conventional joints of meat. But as Ib Pedersen, a Danish pig farmer, lifts them carefully out of the freezer it becomes apparent they are in fact whole piglets - some horribly deformed, with growths or other abnormalities, others stunted. This is the result, Pedersen claims, of feeding the animals a diet containing genetically modified (GM) ingredients. Or more specifically, he believes, feed made from GM soya and sprayed with the controversial herbicide glyphosate. Pedersen, who produces 13,000 pigs a year and supplies Europe's largest pork company Danish Crown, says he became so alarmed at the apparent levels of deformity, sickness, deaths, and poor productivity he was witnessing in his animals that he decided to experiment by changing their diet from GM to non-GM feed. The results, he says, were remarkable: "When using GM feed I saw symptoms of bloat, stomach ulcers, high rates of diarrhoea, pigs born with the deformities ... but when I switched [to non GM feed] these problems went away, some within a matter of days." The farmer says that not only has the switch in diet improved the visible health of the pigs, it has made the farm more profitable, with less medicine use and higher productivity. "Less abortions, more piglets born in each litter, and breeding animals living longer." He also maintains that man hours have been reduced, with less cleaning needed and fewer complications with the animals.