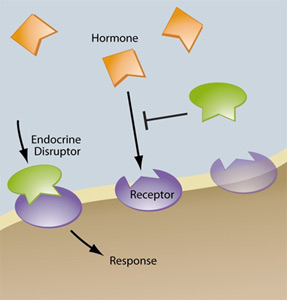
Rising levels of cancers and fertility problems have attracted scientists’ attention to endocrine disrupting chemicals, with some calling for strict regulation of the substances, in line with the precautionary principle. Others meanwhile, stress the worthiness of those chemicals in everyday products such as plastics and warn that the foundations of science risk being turned upside down if precautionary measures are taken. Endocrine disruptors are suspected of triggering diseases such as cancer or diabetes and contributing to people becoming overweight or infertile. The controversy has gone beyond the traditional confrontation between the chemicals industry and environmental and health activists. Among scientists, too, opinions vary on whether a link exists between these chemicals and diseases. But all parties agree on one point – that the protection of consumers and the environment must be ensured. Henk Tennekes comments that a new risk assessment is needed to evaluate the effects that chemicals have on humans and the environment and that endocrine disruptors may be a case in point.
The European Union has been at the forefront of efforts to regulate those substances, for example by banning the use of Bisphenol A in baby bottles, or trying to reduce the amount of pesticides used by farmers. Most importantly, EU lawmakers adopted in 2006 the REACH regulation, which for the first time requested chemical producers to prove that their products are safe before they can be allowed on the market.
The European Commission has now started a review of endocrine disruptors and is expected to submit proposals in 2015 on how to identify and regulate substances with endocrine disruptive properties in products such as pesticides and biocides.
Source: EurActiv, 12 December 2013
http://www.euractiv.com/science-policymaking/endocrine-disruptors-harmf…
- Log in to post comments

Current Risk Approaches Are Flawed
Traditional risk assessments of chemicals are failing to protect human health and the environment. Regulatory decisions based on current risk approaches are, therefore, flawed simply because the science underpinning the risk of chemicals is inappropriate in many cases. A fundamental problem is to use one methodology for all compounds, irrespective of their toxic mode of action in organisms. Empirical evidence demonstrates that the Druckrey–Küpfmüller toxicity model, which was validated for genotoxic carcinogens in the early 1960s, is also applicable to a wide range of non-genotoxic compounds. According to this model, the character of a poison is primarily determined by the reversibility of critical receptor binding. Chemicals showing irreversible or slowly reversible binding to specific receptors will produce cumulative effects with time of exposure, and whenever the effects are also irreversible they are reinforced over time; these chemicals have time-cumulative toxicity.
While most toxicants with a generic mode of action can be evaluated by the traditional concentration–effect approaches, a certain number of chemicals, including carcinogens, methylmercury, rodenticides, neonicotinoids and cartap insecticides have toxic effects that are reinforced with time of exposure, i.e. time-cumulative effects. Therefore, the traditional risk approach cannot predict the impacts of the latter chemicals in the environment. The traditional approach to toxicity testing is to consider dose (concentration):effect relationships at arbitrarily fixed exposure durations, which are supposed to reflect ‘acute’ or ‘chronic’ time scales. This approach measures the proportion of all exposed individuals responding by the end of such exposure times. This is valid when toxicity is mainly dependent on exposure concentrations, but it is insufficient when toxic effects are reinforced by exposure time, because the impact of low exposure concentrations may be underestimated if the duration of the experiment is shorter than the latent period for toxicity. Toxicological databases established in this way are collections of endpoint values obtained at fixed times of exposure. As such these values cannot be linked to make predictions for the wide range of exposures encountered by humans or in the environment. By contrast, Time To Effect (TTE) approaches provide more information on the exposure concentrations and times needed to produce toxic effects on tested organisms. Indeed, TTE bioassays differ from standard chronic toxicity tests in that TTE approaches record effects at consecutive times during the exposure, so the data form a matrix that can be analyzed to extract information about the effective concentrations (e.g. NEC, EC10, LC50, etc.) or about the time to effect for a given endpoint (e.g. t50). This is an essential requirement for risk assessment of chemicals showing time-dependent toxicity, particularly for those that have time-cumulative toxicity, as it allows prediction of toxic effects for any combination of concentration and time found in the environment. Such a new risk assessment is needed to evaluate the effects that time-dependent chemicals have on humans and the environment. Endocrine disruptors may be a case in point.